
Research News
Research News
A novel mechanism of host HEXIM1 protein in promoting an-alpha herpesvirus infection
Recently, Anchun Cheng and Ying Wu’s research group, from the Research Center for Avian Diseases of the College of Veterinary Medicine, published the article "An alpha-herpesvirus employs host HEXIM1 to promote viral transcription" in the Journal of Virology (link:https://pubmed.ncbi.nlm.nih.gov/38363111/). In this study, the authors first found the mechanism of HEXIM1 in AnHV-1 (an avian alpha-herpesvirus) transcription, which enriched and perfected the possible mechanism of α-herpesvirus hijacking host transcription machinery to transcribe viral genes.
Herpesviruses are known to exploit host transcription factors as a means of transcribed viral genes; nevertheless, the ways in which these viruses accomplish this are clearly distinct from one another. Being a universal positive transcription factor for the majority of biological genes, P-TEFb is essential to the biology of viruses. As a major negative regulator of P-TEFb, HEXIM1 is crucial to the equilibrium of P-TEFb recruitment and dissociation. Abnormal P-TEFb expression or modifications to its self-regulation function may result in P-TEFb dysregulation and impact the gene's.
This study revealed that, in contrast to HSV-1 infection, HEXIM1 was considerably increased during AnHV-1 infection. And the gene expression, RNA polymerase II recruitment, and the production of AnHV-1 progeny viruses were all influenced by the overexpression of HEXIM1, which also facilitated the development of the HEXIM1-P-TEFb complex and the loss of RNAPII S2 phosphorylation. In contrast, overexpression of HEXIM1 decreased the expression of several genes linked to host survival, including SOX8, CDK1, MYC, and ID2.Subsequent investigation showed that the AnHV-1 US1 gene triggered HEXIM1 promoter activity through its C-terminus, in contrast to the mechanism used by its homolog HSV-1 ICP22.Moreover, HEXIM1 overexpression was able to partially compensate for the viral proliferation deficiencies induced by US1 deletion early in infection, indicating that HEXIM1 was the cause of AnHV-1's transcriptional advantage when competing with cells. When considered collectively, our findings uncover a novel AnHV-1 transcription process that is dependent on HEXIM1, which has not been documented in earlier herpesvirus or even DNA virus research.
The first author of this article is Associate Professor Ying Wu from Avian Disease Research Center of the College of Veterinary Medicine; the joint first author is PhD candidate Anyang Sun; and the corresponding author is Professor Cheng Anchun. Funding for the study came from the China Agricultural Research System (CARS-42-17), the Sichuan Veterinary Drug Innovation Team Project (SCCXTD-2021-18), and the Natural Science Foundation of Sichuan Province (2022 NSFSC0077).
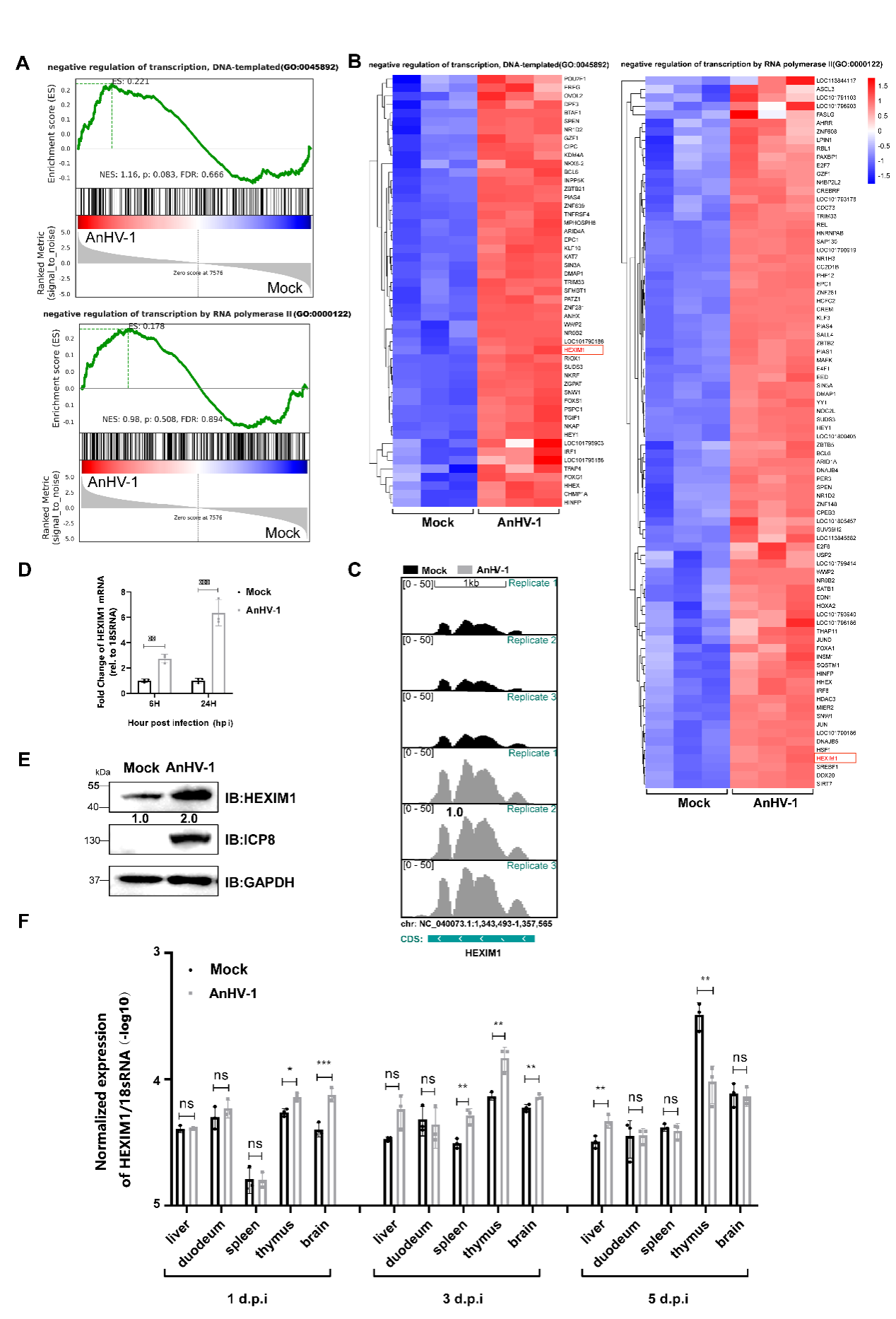
Figure 1. Duck plague virus can regulate the expression of HEXIM1 in vivo and in vitro
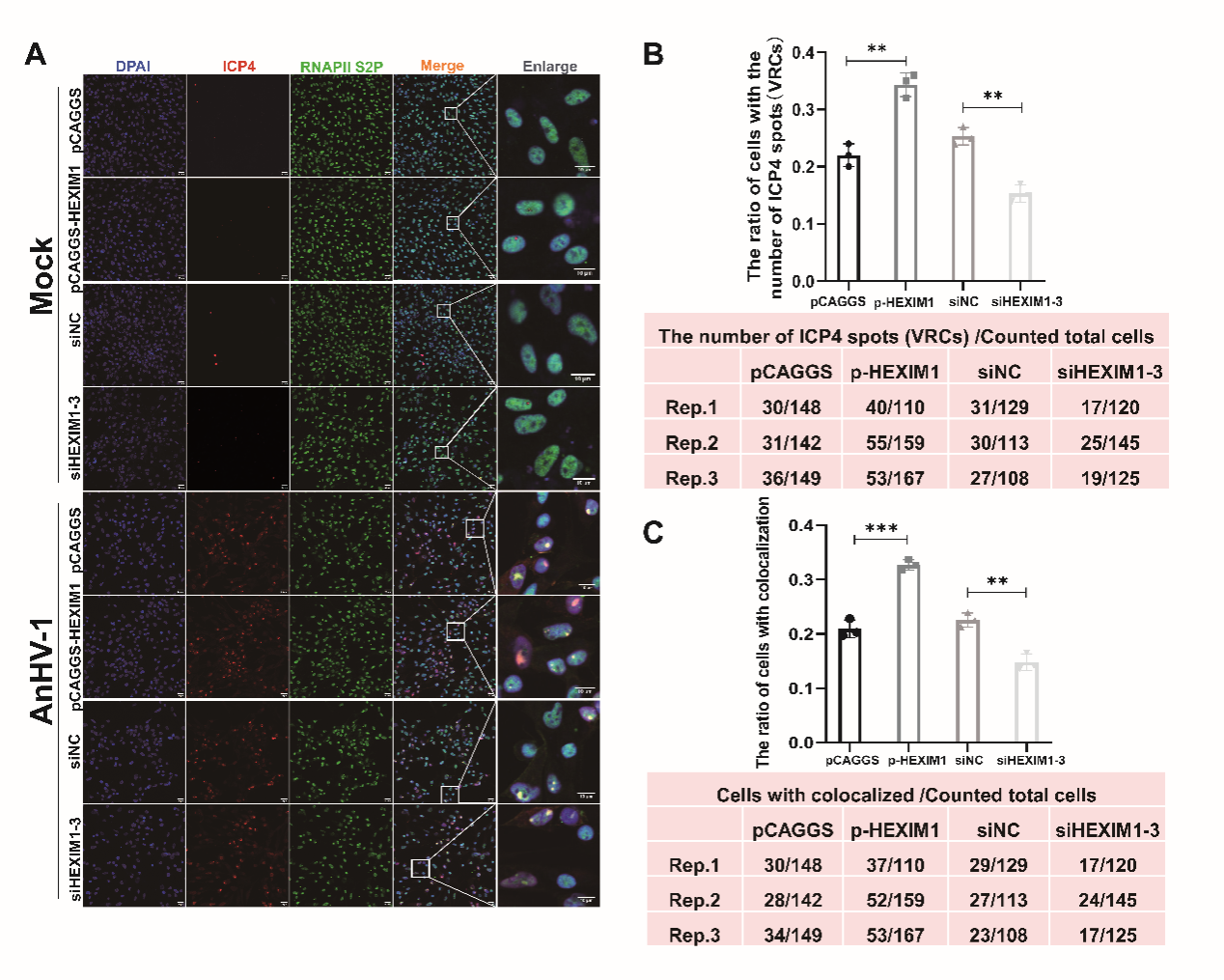
Figure 2. HEXIM1 promotes RNAPII S2P recruitment to ICP4 loci




 028-86296382
028-86296382  No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province
No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province 