
Research News
Research News
Microbiome | Multi-Omics Investigation Revealed the Impact of Long-Distance Road Transportation on Calf Immune and Respiratory Health
On November 16, a significant breakthrough in the field of beef cattle transportation stress was achieved by Professor Zuo Zhicai's team from the Department of Clinical Veterinary Medicine and the National Beef Cattle and Yak Industry Technology System. Their research, titled"Multi-omics Investigation into Long-Distance Road Transportation Effects on Respiratory Health and Immunometabolic Responses in Calves,"was published inMicrobiome, an internationally recognized journal in the field of microbiology. The journal has a current Impact Factor (IF) of 13.8, with a five-year IF of 17.9, and is indexed in the SCI database.
This study utilized a combination of clinical scoring systems and multi-omics analysis, such as 16S rRNA microbiomics, whole-blood transcriptomics and serum metabolomics to evaluate the respiratory microbiome and immunometabolic status of beef calves subjected to a long-distance road transportation.The research confirm that 3000 km of road transportation significantly alters the composition and gene expression profiles of circulating white blood cells in calves, affects their metabolic processes, disrupts the balance of the respiratory microbial community, and leads to pronounced respiratory symptoms that persist for at least 60 days. During this period, the influenced composition and gene expression of circulating blood cells, metabolic processes, and nasopharyngeal microbial community gradually return to equilibrium, and the respiratory symptoms gradually diminish. This study elucidates the systemic effects of road transportation on calves, providing a strong basis for optimizing feeding and management strategies to prevent post-transportation issues in calves, which is of great significance for promoting the healthy development of the beef cattle breeding industry in China.

Fig.1 Overview of experimental design.
Background
Long-distanceroad transportation is a common practice in the beef industry, frequently resulting in bovine respiratory disease (BRD) and compromised growth performance.It is estimated that BRD caused by transportation stress results in direct economic losses of approximately 2.3 billion yuan annually, severely hindering the development of beef cattle industry in China. In recent years, research on the mechanisms of BRD induced by transportation stress and its preventive measures has become a key focus both domestically and internationally. However, a comprehensive investigation integrating clinical performance, physiological conditions, and nasopharyngeal microflora remains lacking.
The rapid advancement in high-throughput sequencing technology and the associated decrease in costs have made multi-omics analyses increasingly popular for investigating physiological and pathological alterations in cattle. For example, the genetic background of BRD infection can be investigated through a multi-omics analysis involving genomics, transcriptomics, and metabolomics.However, existing studies have primarily focused on short-term effects within the first-month post-transport on calves by examining a limited set of indicators. Therefore, our investigation aims to evaluate the multifaceted effect of long-distance road transportation on calves during the first 60 days post-arrival. This includes clinical assessments, routine blood tests (RBT), hematological responses, nasopharyngeal microbiome variations, whole blood transcriptome alterations, and serum small molecular metabolome changes. Our results will provide insights into the extended and comprehensive consequences of road transportation on calf respiratory health and contribute to enhancing post-transport care and management practices.
Research Findings
Collectively, our results confirm that 60 days of road transportation significantly alter the composition and gene expression profiles of circulating blood cells in calves, affect their metabolic processes, disrupt their nasopharyngeal microbial communities, and result in pronounced respiratory symptoms that persist for at least 60 days. During this period, these symptoms gradually diminish, the disrupted nasopharyngeal microbial communities gradually return to equilibrium, and the abundance of BRD-related pathogens gradually decreases. The counts of white blood cells in the circulatory system recover, and the expression levels of stress-related genes are downregulated. Meanwhile, the metabolic processes shift from a catabolic to an anabolic state, characterized by increased concentrations of anabolic-related metabolites and enzyme activities. These alterations are correlated, and several indicators, such as respiratory scores, eosinophil levels, IL-6, glucose concentrations, and pyruvate kinase activity, can serve as markers of respiratory health in calves.
The results indicate that transportation stress, such as fear, hunger, and dehydration, significantly alters the composition and gene expression profiles of white blood cells, as well as the profiles of serum metabolites. These changes adversely affect the performance of calves and disrupt the balance of their nasopharyngeal microflora. Exposure to unfamiliar individuals and new environments further destabilizes the nasopharyngeal microflora equilibrium in calves. This disruption not only affects the white blood cell composition and gene expression profiles but also alters the serum metabolites profiles, culminating in diminished calf performance.
Effective management measures are crucial for mitigating these effects. Supplementing electrolytes and immune agents can enhance the immune function of calves, improve clinical symptoms, and restore the balance of the nasopharyngeal microbiome. The use of antibiotics and probiotics also helps maintain microbial balance. These measures reduced the negative impact of transportation stress on leukocytes and serum metabolites, improving the calves’ overall performance. These findings highlight the extensive and fundamental impact of long-distance road transportation on the respiratory health of beef calves, enhancing our understanding of BRD pathogenesis and providing a scientific basis for developing effective prevention and control strategies.
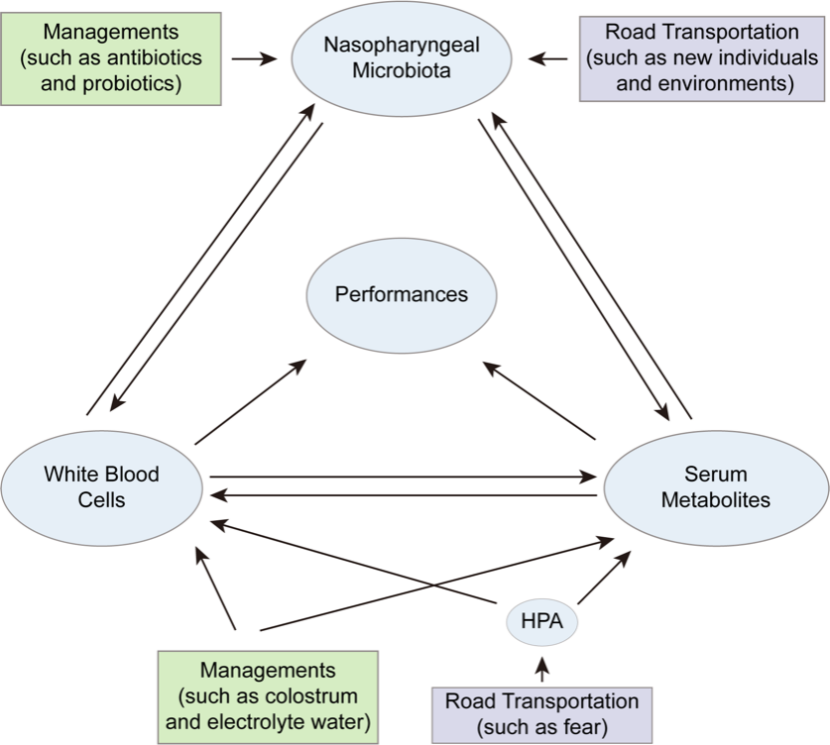
Fig.2 Interactions among white blood cells, serum metabolites, and nasopharyngeal microflora in transported calves.
Based on these insights, we propose 3 strategies to reduce the adverse effects of transportation on calves: minimizing stress sources, blocking signal transmission, and mitigating stress-induced consequences. To minimize stress sources, it is essential to avoid psychological stimuli such as handling, bumps, and noise during transport. Additionally, exposure to pathogenic microorganisms can be reduced by enhancing ventilation systems in transport vehicles. To block signal transmission, stress-induced hormones, oxidative stresses, and inflammatory factors can be diluted or neutralized by supplementing electrolytes and immune agents. This approach reduces their impact on respiratory epithelial tissues through peripheral circulation, thereby preventing cytokine storms and tissue damage. Finally, to mitigate the consequences of stress, pathogenic microorganisms that invade the body through damaged epithelium can be eliminated, and the inflammatory response can be reduced by administering antibiotics and providing anti-inflammatory and antioxidant drugs. The above research reveals that the health of beef cattle raised for fattening in different locations is closely related to scientific feeding management before and after transportation and transportation process control.
Research Team and Acknowledgements
The study was co-led by Professor Fang Jing and Professor Zuo Zhicai from Sichuan Agricultural University, with doctoral students Qi Jiancheng, Huang Fangyuan, and Gan Linli as co-first authors. This investigation was funded by the National Key Research and Development Program of China (2022YFD1601600) and the China Agriculture Research System, a joint venture of the Ministry of Finance and the Ministry of Agriculture and Rural Affairs (Beef Cattle/Yak, CARS-37).
https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-024-01962-2




 028-86296382
028-86296382  No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province
No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province 