
Research News
Research News
Sichuan Agricultural University and Fujian Normal University collaborate to elucidate the structure and mechanism of a novel haemophore
Recently, a research paper titled “Structural Basis and Mechanism of a Unique Haemophore in the Haem-iron Acquisition byRiemerella anatipestifer”was published inAdvanced Science. The paper was a completed by scientists from the National Agricultural (Waterfowl) Industry Technology System, the research team of Professors Anchun Cheng and Mafeng Liu from Sichuan Agricultural University, and the research group of Professor Songying Ouyang from Fujian Normal University.
Iron is essential nutritional element required by most bacteria for growth and metabolism, playing roles in numerous essential life processes. In vertebrate hosts, haem serves as the most abundant iron reservoir, with approximately 70% present in the form of hemoglobin. To acquire haem, Gram-negative bacteria rely on haem uptake systems, which consist of haemophore, TonB-dependent haem receptors, the TonB complex, and ABC transporters. To date, five distinct types of haemophores have been identified; however, no similar sequences or structures have been found in the orderFlavobacteriales.In this study, a novel haemophore, RhuH, was discovered inR. anatipestifer. This protein exists as a dimer (Fig. 1a–c), and its monomer is composed of 7 α-helices and a β-barrel structure formed by 13 anti-parallel β-strands (Fig. 1d). This structure is significantly different from other known haemophores, and RhuH homologs are widely distributed in the orderFlavobacteriales, where these homologous proteins are highly conserved in both structure and function.
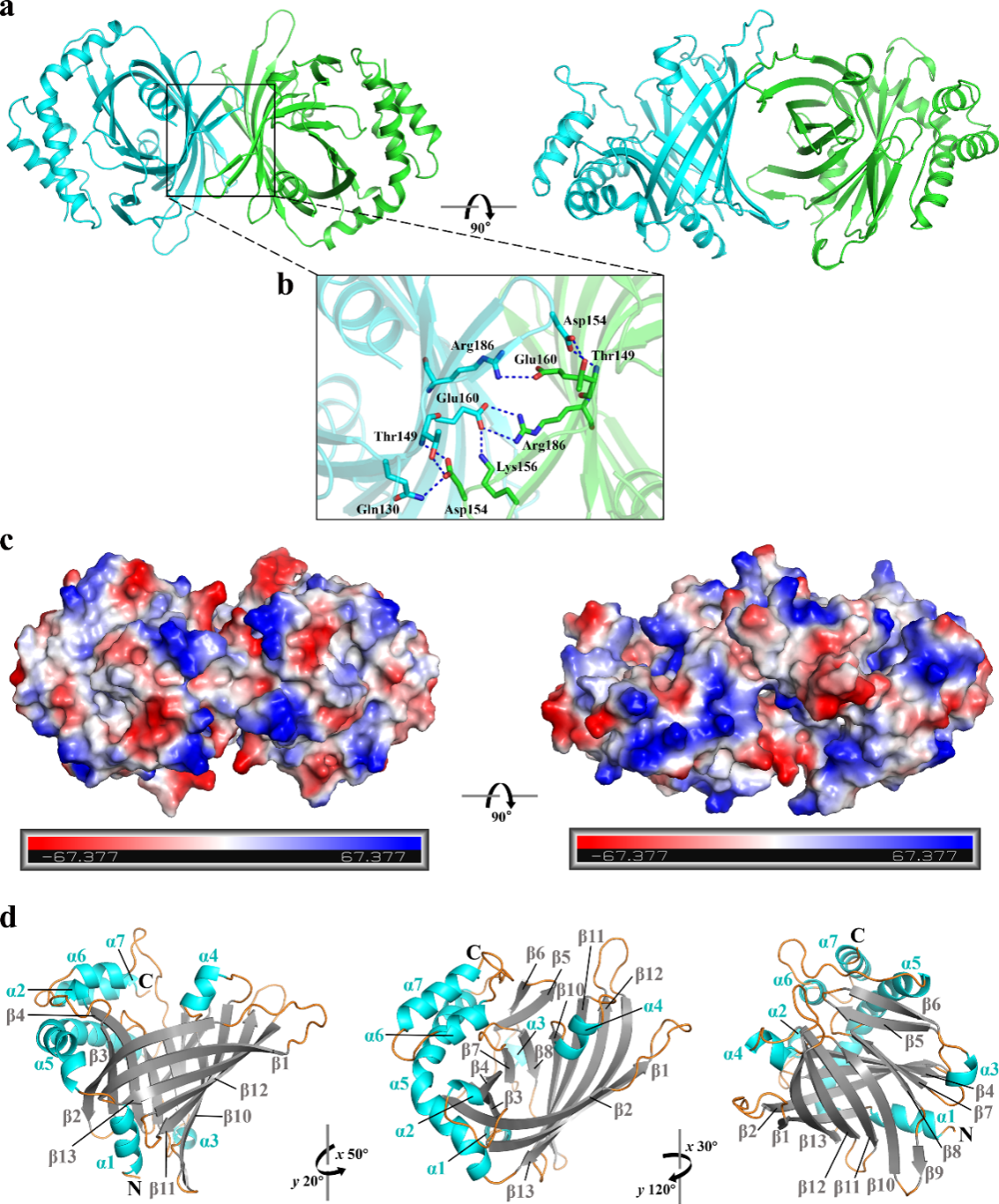
Figure 1. Three-dimensional structure of RhuH
(a) Dimer structure of RhuH. (b) Enlarged view of the interactions at the dimer interface. (c) Electrostatic surface potential of RhuH protein, with blue representing positive and red representing negative charges. (d) RhuH monomer structure displayed from different angles.
Under iron-limited conditions, RhuH is secreted via outer membrane vesicles (OMVs) and can extract haem from host hemoglobin with high affinity in two possible ways (Fig. 2):(a)RhuH is released following the lysis of OMVs secreted by the bacteria; it then extracts haem from host hemoglobin and transfers the bound haem to the corresponding outer membrane haem receptor;(b)RhuH, which is positioned on the surface of OMVs, directly extracts haem from host hemoglobin, after which the vesicle transports the haem back into the bacterial cell via membrane fusion for utilization.

Figure 2. Schematic diagram illustrating the action mode of the haemophore RhuH
Dr. Mengying Wang from the College of Veterinary Medicine at Sichuan Agricultural University and Dr. Dandan Zhang from the College of Life Sciences at Fujian Normal University served as co-first authors of the paper. The co-corresponding authors are Professor Songying Ouyang of Fujian Normal University, along with Professors Anchun Cheng and Mafeng Liu from the College of Veterinary Medicine at Sichuan Agricultural University. Additionally, undergraduate Yizhou Yao from the "Dengfeng Program" contributed to the research. This research team focused on the study the interactions betweenR. anatipestiferand host nutritional immunity. The study was supported by projects including the National Natural Science Foundation of China (32172851), the Natural Science Foundation of Sichuan Province (2024NSFSC0034), and the earmarked fund for China Agriculture Research System (CARS-42-17).
The article link: https://doi.org/10.1002/advs.202412202




 028-86296382
028-86296382  No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province
No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province 