
Research News
Research News
Research Provides New Strategy for Treating Bacterial Biofilm Infections
Recently, the team led by Cheng Anchun and Xu Funeng from the Animal Medical Immunology Institute at Sichuan Agricultural University’s College of Veterinary Medicine, who also serve as experts for the National Modern Agricultural Industry (Waterfowl) System’s Immunosuppressive Disease Prevention and Control Program, published a research paper in the Nature Index journalAdvanced Functional Materials(impact factor: 18.5). The study designed and constructed a nitric oxide (NO) nano-producer capable of capturing endotoxins, which synergizes with photodynamic therapy (PDT) to effectively treat bacterial biofilm infections.
The overuse of antibiotics in humans and animals has led to severe bacterial resistance, particularly in biofilm-associated infections, which account for over 80% of chronic and clinical diseases. Bacterial biofilms form a protective barrier through extracellular polymeric substances (EPS), such as polysaccharides, proteins, and extracellular DNA, which shield bacteria from immune clearance and impede antibiotic penetration. Additionally, the hypoxic and acidic microenvironment within biofilms further diminishes antibiotic efficacy. Effective treatment of biofilm infections requires simultaneous disruption of EPS to release bacteria into a planktonic state, dispersion of the biofilm structure, and complete bacterial eradication. Incomplete treatment risks accelerating bacterial dissemination and exacerbating infections due to enhanced virulence.
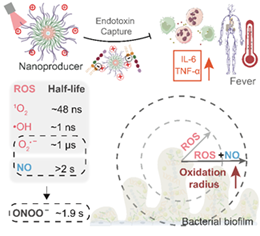
Figure 1. Schematic of the acid-sensitive ROS/NO nanoproducer enhancing action radius and therapeutic efficacy
Photodynamic therapy, an emerging approach, generates reactive oxygen species (ROS) to induce oxidative damage, offering potential for biofilm disruption and bacterial elimination. However, the short half-life of ROS limits their oxidative range, reducing therapeutic efficacy. Moreover, in clinical settings, mixed bacterial infections are common, and ROS-induced oxidative stress may trigger endotoxin release, leading to inflammation, fever, and other complications. Thus, enhancing ROS’s oxidative range while mitigating endotoxin-related risks remains a critical challenge in biofilm therapy.
This study innovatively developed a nano-producer that combines NO with ROS, extending ROS half-life by up to a million-fold and generating peroxynitrite (ONOO⁻), a more potent oxidant. Additionally, the nano-producer incorporates pH-sensitive polymer segments that undergo protonation in the biofilm’s mildly acidic microenvironment, reversing their charge to adsorb negatively charged endotoxins and reduce health risks. This oxidative damage-based approach theoretically eliminates diverse bacterial biofilms without inducing resistance, offering a promising solution for treating drug-resistant bacterial biofilm infections that threaten China’s duck farming industry.
Paper information:https://doi.org/10.1002/adfm.202418928




 028-86296382
028-86296382  No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province
No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province 