
Research News
Research News
PLOS Pathogens | Revealing the Dual Receptor-Targeting Mechanisms of Salmonella Lambda-Like Phage Gifsy-1
Recently, the team of Cheng Anchun/Zhao Xinxin from the Institute of Veterinary Immunology, College of Veterinary Medicine, Sichuan Agricultural University, who is also the Expert in the Prevention and Control of Immunosuppressive Diseases at the National Modern Agricultural Industry (Waterfowl) Technology System, published a research paper entitled "Two receptor-targeting mechanisms of lambda-like siphophage Gifsy-1 of Salmonella Typhimurium" in PLOS Pathogens, an authoritative international journal in microbiology and pathogenesis. The study systematically clarified the molecular mechanisms by which the temperate phage Gifsy-1 infects smooth and rough Salmonella strains, revealing two distinct yet flexibly switchable receptor-targeting strategies mediated by its side tail fiber protein Stf and central tail tip protein J. This provides a crucial theoretical basis for understanding phage-host interactions and developing phage therapies against drug-resistant Salmonella.
Antimicrobial resistance (AMR) poses a major threat to global public health, with infections caused by multidrug-resistant Salmonella being particularly concerning. As a novel strategy to combat drug-resistant bacterial infections, phage therapy hinges on a precise understanding of the molecular mechanisms by which phages recognize and infect host bacteria. The lipopolysaccharide (LPS) O-polysaccharide (OPS) layer on the surface of Salmonella acts as a physical barrier to many phages, and Salmonella can switch between smooth (intact OPS) and rough (OPS-deficient) LPS phenotypes through phase variation in natural environments to adapt to environmental changes. However, the mechanism by which phages adapt to changes in host OPS to successfully establish infection remains unclear.
The study focused on Gifsy-1, a widely distributed and highly conserved temperate phage in Salmonella. First, it was confirmed that Gifsy-1 has a broad host range, capable of lysing multiple Salmonella serotypes, but its infection efficiency is higher in rough strains, indicating that OPS is a barrier to its infection. In-depth research revealed that Gifsy-1 can flexibly switch between two different receptor-targeting mechanisms depending on whether the host is smooth or rough (see the figure below): 1) For rough strains: Gifsy-1 adopts a multi-receptor synergistic targeting mode: the side tail fiber protein Stf targets the galactose II (Gal II) group on the core oligosaccharide (COS) located beneath the OPS layer and linked to the outer membrane, while the central tail tip protein J simultaneously targets three outer membrane proteins—OmpC, OmpX, and BtuB. Among these, OmpC has a unique dual function: it serves as both the primary receptor and the secondary receptor necessary for triggering DNA injection; 2) For smooth strains: the OPS layer blocks Gifsy-1 from recognizing outer membrane protein receptors. At this time, the phage uses COS Gal II recognized by Stf as the sole receptor to complete adsorption and DNA injection. The innovation of this study lies in revealing that lambda-like siphophages adapt to host OPS phase variation through two receptor-targeting mechanisms, a characteristic that may be an important reason for the widespread distribution of Gifsy-1.
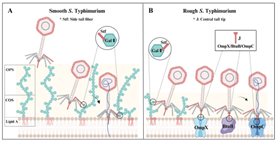
Figure: Two receptor-targeting mechanisms of phage Gifsy-1 infecting smooth and rough Salmonella Typhimurium
Zeng Xiaoli, a doctoral student from the Institute of Veterinary Immunology, College of Veterinary Medicine, Sichuan Agricultural University, is the first author of the paper, and Professors Cheng Anchun and Zhao Xinxin are the corresponding authors. The research was supported by the National Key R&D Program of China (2023YFD1800200), the National Natural Science Foundation of China (32072877), the Sichuan Veterinary Drug Innovation Team (SCCXTD-2024-18), and the National Modern Agricultural Industry Technology System Special Project (CARS-42-17).
Paper link: https://doi.org/10.1371/journal.ppat.1013352




 028-86296382
028-86296382  No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province
No. 211 Huimin Road, Wenjiang District, Chengdu, Sichuan Province 